 Johannes F. Plate, MD*; Ali Mofidi, MB, BAO, BCh, M.Med., Sci., FRCSI, FRCS Orth**; Sandeep Mannava, MD, PhD*; Cara M. Lorentzen, MD*; Beth P. Smith, PhD*; Thorsten M. Seyler, MD*; Timothy McTighe, Dr. HS (hc)*** and Riyaz H. Jinnah, MD, F.R.C.S* Johannes F. Plate, MD*; Ali Mofidi, MB, BAO, BCh, M.Med., Sci., FRCSI, FRCS Orth**; Sandeep Mannava, MD, PhD*; Cara M. Lorentzen, MD*; Beth P. Smith, PhD*; Thorsten M. Seyler, MD*; Timothy McTighe, Dr. HS (hc)*** and Riyaz H. Jinnah, MD, F.R.C.S*
Abstract:
compartment osteoarthritis of the knee. Despite the many years of experience performing UKA, the orthopaedic community has not reached a consensus on the patient selection criteria or operative indications for UKA, due to varied outcome results in the literature. Newly designed robotic-assisted systems are believed to increase the precision and accuracy with which unicompartmental knee arthroplasty can be performed, possibly leading to fewer mechanical failures and improved functional outcomes. However, long-term follow-up is required before definitive conclusions can be reached regarding this new technology. This review examines the history of UKA, reviews early results of robotic-assisted UKA and presents an outlook on future advances.
Key Words: unicompartmental knee arthroplasty, total knee, robotic assisted
* Department of Orthopaedic Surgery, Wake Forest School of Medicine, Medical Center, Boulevard, Winston-
Salem, NC 27157-1070, USA
** Morriston Hospital Swansea SA6-6NL, UK
*** JISRF, Chagrin Falls, Ohio www.jisrf.org
Correspondence to:
Riyaz H. Jinnah, MD, FRCS
Department of Orthopaedic Surgery
Wake Forest School of Medicine
Medical Center Boulevard
Winston-Salem, North Carolina 27157-1070
Phone: 336-716-8200
Fax: 336-716-6286
Email: rjinnah@wakehealth.edu |
History of Unicompartmental Knee Arthroplasty
The theory of unicompartmental replacement of only one side of one compartment of the knee joint came from Duncan C. McKeever in the 1950s, followed up by both McKeever and MacIntosh introducing metallic tibial components that resurfaced only the tibial plateau.[1]

MacIntosh Component / McKeever Component
Metallic tibial resurfacing components implanted in the late 1950s and through the 1960s were fraught with high complication rates and unacceptable functionality.[1]
Frank Gunston and the Charnley Connection
A young Canadian surgeon (Dr. Frank Gunston) from Winnipeg, Manitoba, Canada on a traveling fellowship to study hip arthroplasty at Wrightington became intrigued with the ongoing problems associated with arthritic knees. During his fellowship, Gunston developed a design for knee arthroplasty refl ecting his exposure to UHMWPE and hip arthroplasty.[1]
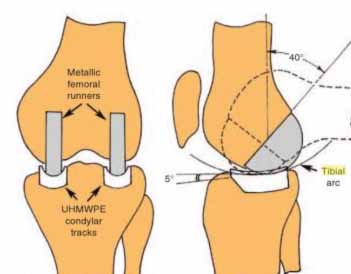
Illustration of Polycentric TKA design by F. Gunston 1969
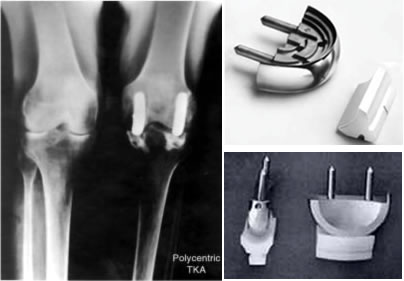
Polycentric Radiographic Post-op view and product
John Charnley had no active role in Gunston’s knee work, however, Charnley did develop his own unicompartmental knee that was introduced and distributed by Thackeray as the Load Angle Inlay.[1]
This featured a convex UHMWPE femoral component articulating against a fl at metallic plateau.[1] Charnley’s design did not survive due to loosening, deformation and wear of the plastic femoral component. However, the tibial (metal) components stood up remarkably well.[2,3]
Charnley's Load Angle Inlay Total Knee by Thackeray, Ltd.
1974 Bechtol Patellofemoral Component |
During UKA, one tibiofemoral compartment is resurfaced in order to reduce deterioration of the joint space and to eliminate resultant pathological joint biomechanics.[4,5,6] The medial compartment is most commonly affected by degenerative changes and treated with UKA, followed by the lateral and patellofemoral (PFJ) compartments.6 Bechtol introduced the first total patellofemoral component back in 1974 which introduced the concept of resurfacing both sides of the PFJ. Subsequently in 1976 Blazina designed an extension of the trochlear component that extended toward the intercondylar notch (Type II). Following these developments, Blazina and associates published the first report of patellofemoral resurfacing.[7,8] However, all compartments can be resurfaced independently.[5]
Historically, UKA was associated with varied clinical results. Although the surgery was a commonly performed procedure in the 1970’s and 1980’s, its popularity faded as a result of both large numbers of patients ultimately required revision surgery with conversion to total knee arthroplasty (TKA)[5,9,10,11] and the controversy between Marmor and Richards Manufacturing Co.[12]
In the period from 1970 through 1972, Dr. Leonard Marmor, an orthopedic surgeon working with the Richards Manufacturing Corporation, developed a prosthetic device known as the Marmor Modular Knee. During 1973, Richards, through engineering error, began manufacturing final metal components of the medium category which were larger than originally designed and which therefore did not match the medium template and trial components. It was thus possible that a surgeon could prepare a bone for insertion of the medium metal component using properly sized medium template and trial components (which were reused from surgery to surgery) and then cement into place a toolarge medium final component. The apparent mismatch and controversy over this issue became public knowledge with announcement letters to the orthopaedic community by both Dr. Marmor and Richards Manufacturing Co. in 1975. The medical-legal action that followed scared many users of the Marmor Modular Knee to abandoned this procedure for fear of legal entanglement.[12,13,14] This slowed down clinical surgical evaluations of unicompartmental total knee arthroplasty for a decade.
Marmor Modular Knee Ad from JBJS 1970s
With the advancement of improved technology, a more comprehensive understanding of joint biomechanics and an increased desire for minimally invasive surgery, UKA has undergone a recent resurgence in popularity for the management of degenerative changes of the knee joint.[4,5,10,15]
Early Results

Marmor Modular Knee |
In the early 1970’s Marmor introduced a modular UKA implant (“Marmor Knee).[16,17] The Marmor Knee adopted the resurfacing concept and addressed both compartments of the knee. However, Marmor subsequently resurfaced only a single side of the knee. The prosthesis was unconstrained included an all-polyethylene inlay tibial component and a narrow femoral component with a single peg.[18]
In 1976, Marmor reported on 105 patients with a minimum of 2 years of follow-up implanted with the Marmor Knee.[19] Successful results with functional improvement and a stable articulation were achieved in 88% of patients. After 10 to 13 years of follow-up, patients implanted with the “Marmor knee” during this period maintained satisfactory results in 70% of cases and 86.6% of patients remained pain free.[20,21] Marmor noticed subsidence of the relatively small tibial component causing early failure.[20,21] There was also an increased risk of wear and loosening (due to cold fl ow and deformation) of the 6mm polyethylene component leading to revision surgery. Marmor then recommended the use of a thicker polyethylene component.[20,21] In the mid-1980’s, the Marmor Knee was available with metal-backing to eliminate creeping and cold fl ow, which was found to be a problem with the early all-polyethylene design.[22]
Considering Marmor’s contributions and innovation to UKA component design and operative technique, he is regarded by many as the godfather of modern UKA. Unfortunately, a patent controversy with Zimmer and contract disputes with Richards over changes of the original Marmor Knee design overshadowed the early success of this prosthesis and may have hampered the wide-spread use of UKA in the following years.[12,13,14]
Insall et al. reported results from 32 UKA procedures in 1980.[11] Despite losing ten patients to follow-up in the first year, the study showed no change in the mean Hospital for Special Surgery knee score of 48 points between pre- and postoperative assessment. Following UKA, varus angulation had decreased from a preoperative mean of 8° to 4° postoperatively and valgus angulation had decreased from a preoperative mean of 21° to 8° postoperatively. The final clinical outcomes of the 22 UKA performed during the study varied widely: excellent (one knee, 5%); good (seven knees, 32%); fair (four knees, 18%); and poor (ten knees, 45%). Although correction of the anatomical alignment was achieved after UKA in the Insall case series, the clinical patient outcomes were unfavorable.
While the Insall series demonstrated poor clinical outcomes after UKA, other case series reported more favorable results. In 1986, Broughton et al. published a retrospective review of 42 UKA, which were rated according to the Baily knee score.[23] In this study, 32 of the total number of 42 knees (76%) were rated as ‘good’, and 24 knees continued to maintain a ‘good’ rating five to six years after the procedure. The remaining eight knees were evaluated seven to ten years after surgery and also maintained a ‘good’ rating. Only seven of all 42 knees (17%) in this study were rated ‘fair’ or ‘poor,’ and three knees (7%) required revision arthroplasty.[23] Similar mid-to-long term clinical results were reported by Bert in a retrospective review of patients undergoing UKA almost a decade later in 1998.4 In this study, post-surgical outcomes showed 87.4% survivorship of the UKA ten years after the procedure. Murray et al. reported on the outcomes of 143 knees treated with medial sided UKA using the Oxford mobile bearing prosthesis between 1982 and 1992.[24] Patients were followed for a mean of 7.6 years postoperatively and revealed a 97% survivorship. Five revisions were reported; two revisions for progression of osteoarthritic disease in the lateral compartment; one for component loosening; one for an infection; and one for a painful prosthesis without any radiographic abnormalities.
Operative Indications for Unicompartmental Knee Arthroplasty
Adhering to the operative criteria for UKA may be critical for surgical success and patient benefit, as improper patient selection is thought to be a risk factor for early UKA failure.[25,26] Classic indications for UKA proposed by Kozinn and Scott and others included: a patient with a sedentary occupation; age of greater than or equal to 60 years; minimal pain at rest, less than 10° varus deformity; range of motion of at least 90° without a fl exion contracture; correctable medial deformity; 50% unicompartmental joint space collapse; weight less than 82 kg; thin body habitus (as obesity is a relative contraindication); diagnosis of osteoarthritis (OA), post-traumatic arthritis or osteonecrosis; and isolated unicompartmental knee pain (Table 1).[4,11,25-31] Furthermore, successful UKA requires an intact anterior cruciate ligament (ACL) and a stable knee that resists femorotibial subluxation.[10,26]
Traditionally, contraindications for UKA included: the diagnosis of rheumatoid arthritis or other infl ammatory arthritic conditions; knee pain in all compartments; decreased range of motion with a fl exion contracture; obesity; knee instability; ACL rupture; and age of less than 60 years.[4,26,28] Insall et al. reported that four out of seven patients (57%) under the age of 60 who received UKA experienced ‘poor’ surgical outcomes, whereas six out of 15 patients (40%) over 60 years of age experienced ‘poor’ results in their case series.[4] Contrary to the Insall et al. series, Berend et al. concluded that failure, which was defined as a UKA requiring later revision or an impending revision, was not associated with age, gender, disease severity or implant design, but with increased body mass index.[32] A body mass index of greater than 32 was predictive of UKA failure and a reduced survivorship. Studies published in the early 1990s also noted that obese patients have a failure rate 1.4 times higher than patients with normal weight.[4,33] Unfortunately, most of the data related to risk stratification for UKA surgery is based upon Level 4 and 5 evidence. The level of evidence coupled with low statistical power in these studies contributes to disagreement and continued controversy in the literature regarding pre-operative UKA patient selection criteria.
Good outcomes for patients initially thought to be outside the UKA operative criteria have been reported in the literature, and these studies have added to the confusion over appropriate UKA operative selection criteria.[26,34] Pennington et al. showed results for 41 patients and 46 knees undergoing UKA, in which all patients were under the age of 60 years.[34] The decision to proceed with UKA was made intraoperatively after direct observation of all three knee compartments. The Hospital for Special Surgery knee score and the University of California at Los Angeles (UCLA) activity assessment were utilized to assess postoperative outcomes. A total number of three knees (6.5%) were revised and one patient (one knee, 2%) was lost to follow-up. In general, the younger patient cohort had favorable clinical outcomes. Specifically, 39 of 42 patients (93%) had ‘excellent’ results and the other three patients (7%) had ‘good’ results, based on Hospital for Special Surgery knee score measurements. The UCLA assessment score for all 42 knees that were not revised was 6.6 ± 1.4. For the three patients that underwent revision, the UCLA assessment score was 7.3 ± 1.5.[34] These findings combined with other recent reports, have expanded the classical indications for UKA, as this surgery has been successfully performed on younger patients. Further long-term evaluation of these cases is necessary to determine how UKA will perform clinically in this expanded patient demographic.[10,27,28,35,36,37]
Although the operative indications have recently been expanded (Table 1), surgeons continue to have trouble with the diagnosis and management of unicompartmental knee pathology.
Stern et al. reviewed 228 knees in need of arthroplasty and found that only 6% (13 knees) fit the operative criteria for UKA.[4,38] Bramby et al. and Laskin reported similar findings, with approximately 15% of their preoperative evaluations for knee arthroplasty meeting the operative criteria for UKA.[4,39] The available evidence from the literature supports that proper preoperative evaluation of unicompartmental knee pain is paramount to operative success and favorable clinical outcomes.
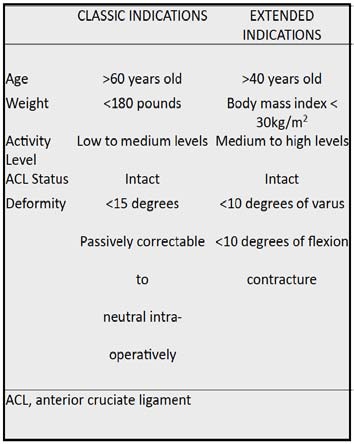
Table 1. Indications for Unicompartmental Total Knee Arthroplasty
Patellofemoral Disease

Contemporary Patella-Femoral Component |
The presence of patellofemoral disease has been traditionally regarded as a contraindication to UKA of the medial or lateral compartment due to the risk of early failure.[31,40,41,42] However, Goodfellow and O’Connor and Beard et al. did not find a correlation between patellofemoral disease and outcomes of UKA and recommended that this contraindication may be disregarded.[43,44] In a recent study, Pandit et al. compared 678 mobile-bearing UKA procedures in which at least one traditional contraindications (anteromedial OA, medial osteonecrosis) was ignored to 322 mobile-bearing UKAs without any contraindications.[42] Clinical and functional outcomes, failure rate, and survival were similar in both groups and the authors suggested that traditional contraindications are not required for mobile-bearing UKA.[42] These findings were confirmed by Berend et al., who concluded that radiographic findings of patellofemoral OA can be safely ignored for mobile-bearing UKAs.[41]
Clinical Evaluation for UKA: The “One Finger Test”
The “one finger test” is a useful way to diagnose unicompartmental knee pain.4 For this test, the patient should be able to localize the joint pain by pointing to the symptomatic area with a single finger.[4,29] If the patient cannot locate the pain with one finger, or grabs the whole knee, UKA may not be indicated.4 After a thorough history and physical examination, further radiographic evaluation is required with standard plain anterior-posterior and lateral films, varus/valgus stress views, and possible Magnetic Resonance Imaging or Computed Tomography scanning. By combining the clinical history, the radiographic studies, and knowledge of the operative indications, the surgeon can determine the risks, benefits and alternatives to performing UKA.
The Use of UKA Today

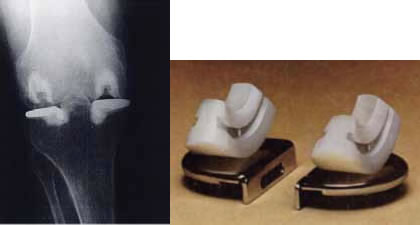
Contemporary Uni Design |
Between 1997 and 2000, UKA comprised approximately 1 – 6% of the knee arthroplasty cases performed in the United States.[45,46] In 2007, almost 45,000 UKA procedures were performed, approximately 8% of all knee arthroplasties. In 2009, the number of UKA further increased to approximately 51,300 cases, and this number is estimated to climb to 55,100 cases in 2010.[47] The number of UKA procedures being performed annually is estimated to grow at a rate of 32.5% per year, which is greater than the 9.4% growth in the number of total knee arthroplasty (TKA) procedures performed annually.[48] However, in a poll at the 2010 Annual Meeting of the American Academy of Hip and Knee Surgeons, 212 of 256 respondents (82.8%) reported that performing UKAs comprised of only 0 - 9% of their practice.
In other countries, the procedure is performed with greater frequency than in the United States. The Norwegian Registry reported that UKA accounted for 11.8 % of the 38,122 knee surgeries performed between 1994 and 2009.[49] Similarly, the Australian registry showed that primary UKA comprised 11.4% of all knee replacement surgeries in that country.[50]
However, UKA could possibly be performed more often, as a large percentage of patients who meet the operative criteria for UKA are not given the surgical option. Often, these patients are treated at centers without the surgical expertise or equipment to offer UKA.[51]
Unicompartmental Knee Arthroplasty versus Total Knee Arthroplasty
Numerous surgical benefits associated with UKA have increased its popularity. These advantages include less perioperative morbidity, reduced blood loss, shorter postoperative recovery and rehabilitation, increased post-surgical range of motion, and reduced surgical costs compared to TKA (Table 2).[4,5,10,25,27,28,52,53]

Table 2. Advantages of UKA compared to total knee arthroplasty
Specifically, a Minnesota registry documented a 2.8 day mean length of hospital stay for 240 UKA cases, compared to a 4.5 day length of hospital stay for TKA.54 The Minnesota registry also documented a mean blood loss of 350 mL during the 240 UKA cases, compared to 613 mL average blood loss in 87 patients undergoing a total TKA described by Hinarejos et al. and a mean blood loss of 1747 mL for 30 TKA patients published by Kalairajah et al.[54,55,56] The data suggest that performing UKA will result in less blood loss when compared to TKA, although these reports represent case series with varying power, performed at different institutions, by separate investigators.
UKA may be a preferable alternative to TKA in selected patients. In 1991, Laurencin et al. followed 23 patients, who received UKA in one knee and TKA in the contralateral knee, for 81 months.[57] The surgeries were performed by the same surgical team during the same hospitalization. Inpatient care and rehabilitation protocol remained the same for both knees throughout the hospital course. Range of motion after UKA increased from an average of 106° preoperatively to 123° postoperatively. The range of motion on the contralateral TKA increased from a mean of 104° preoperatively, to 109-113° postoperatively, depending on whether the patient underwent concurrent patellar resurfacing.[57]
Dalury et al. reported on the outcomes of a cohort of 23 patients who underwent TKA in one knee and UKA in their contralateral side.58 At an average follow-up of 46 months for TKA and 42 months for UKA, patients reported positive results for both surgical interventions. In particular, the UKA patients experienced an increase in the mean postoperative range of motion (123.5° + 9°). These results were comparable to the postoperative range of motion after TKA in the contralateral knee, which was 119.8° ± 7°. There was a similar increase in Knee Society Scores for both procedures, with UKA increasing from 45.9 to 89.7 and TKA knees increasing from 42.4 to 90.3. Despite having comparable outcome measures, 12 out of the 23 patients (52%) in the study expressed a preference for their UKA over TKA. The remaining 11 patients (48%) in the cohort expressed no preference, whereas TKA was not chosen by any patient as the preferred procedure.[58] A report from 2005 surveyed patients who had undergone both UKA and TKA; the majority of study participants stated that the UKA felt more like a “natural” knee.[4]
The subjective feeling of UKA as being more normal compared to TKA can be explained by joint biomechanics. Patil et al. found that tibial axial rotation and femoral rollback more closely resemble normal anatomy in UKA compared to TKA.[59] In addition, UKA is less disruptive to native knee anatomy because only one-third of the knee joint is replaced, the cruciate ligaments remain intact after surgery, and the menisci of the untreated compartment are preserved.[4,27,28]
Dalury et al. and Laurencin et al. offer compelling evidence in support of performing UKA.[57,58] The methodological design of each of the two studies was unique in that the study cohort underwent two different interventions on each of their knees, allowing for an internally controlled case series.
This design allows for less variability in the data, resulting in improved statistical power with fewer subjects. The problem with a repeated measures study design is that the two interventions, UKA versus TKA, cannot be considered truly independent, as they both occurred in the same individual. In these types of studies, it is difficult to separate how one intervention has infl uenced the other.
Besides pain relief, functional recovery remains an important component of operative success after knee arthroplasty. The goals of orthopaedic surgery are to restore normal joint motion, return the patient to full function, prevent further degenerative disease, and provide the patient with an expedited return to work and recreational activities. Hopper et al. conducted a study to determine how easily patients returned to low-impact sports after either UKA or TKA.[60] Patients who underwent UKA returned to sports in a mean time of 3.6 months, compared to 4.1 months for TKA patients. The amount of time spent participating in low impact sports for UKA patients rose from an average of 85 minutes per week preoperatively, to an average of 92.1 minutes postoperatively. Recreational participation time decreased from a mean of 62.7 minutes preoperatively, to a mean of 37.5 minutes per week postoperatively for TKA patients. Pain during sporting activities was experienced by only 24.1% of UKA patients as compared to 42.9% of TKA patients.[60] These studies concluded that patients undergoing UKA returned to sports faster, were able to participate in physical activity for longer periods of time, and had less joint pain with greater knee function.[60,61]
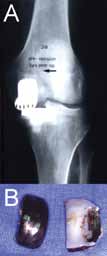 Fig. 2 Wrong component sizing or positioning may lead to edge loading (A) resulting in increased wear and implant failure (B). Fig. 2 Wrong component sizing or positioning may lead to edge loading (A) resulting in increased wear and implant failure (B). |
Limitations Preventing the Widespread Adoption of the Unicompartmental Knee Arthroplasty Procedure in Clinical Practices
While UKA may have advantages as a surgical option for selected patients who meet the operative criteria detailed previously, TKA remains a popular operation for unicompartmental pathology. The widespread performance of UKA has been limited by the technical difficulty of performing the procedure. In particular, UKA has less tolerance for acceptable component positioning when compared to TKA, as improper component positioning, by as little as 2°, can result in UKA failure (Figure 2).[5,37,46,62-68] Failures of UKA occur when there is medial-lateral mismatch, inadequate stability of the components, heterogeneous polyethylene wear, improper patient selection (such as performing UKA for bilateral osteoarthritis), aseptic loosening, and tibial subsidence (Figure 3A and 3B).[4,27]
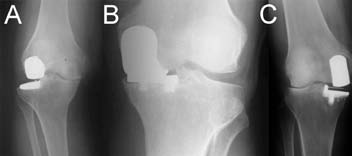
| Fig. 3 Disease progression of the other compartment from overstuffing, over-correction or misbalance (A), early loosening (B) and wrong component positioning may lead UKA failure. |
Fig. 3C |
|
Improper alignment is considered to be the leading cause of UKA failure (Figure 3C).[28,61]
Maligned components often lead to impaired joint biomechanics, and eventual knee pain.[5,69,70] Reports in the literature have associated a technically poor UKA operation with accelerated polyethylene wear, an accelerated progression of the pathology to the contralateral compartment, and, in some rare instances, femoral fracture.[5,28,63,71,72,73] Strict adherence to operative technique and acceptable tolerances are required to maximize the benefits of UKA. Preservation of adequate bone stock is crucial to surgical success, leading to a shorter recovery and rehabilitation time.[25,28,45] Further, excessive bone resection often results in poor tibial component stability, which has been associated with a more difficult conversion to TKA if revision arthroplasty is eventually required.[4,68] The technical demands of performing UKA, coupled with the small margin for error, have limited the widespread adoption of this surgical intervention for unicompartmental knee pathology and many surgeons and patients remain wary of the historically inconsistent post-surgical results published in the literature.
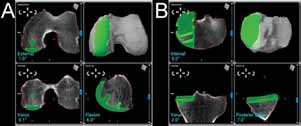
Fig. 4 Intraoperative screenshots of the robotic system showing the computer model of any anatomy based on preoperative CT-scans and allowing for precise positioning of the femoral (A) and tibial (B) components. |
Use of Robotics for Unicompartmental Knee Arthroplasty
Historically, UKA has been considered a technically demanding procedure that poses a challenge to the orthopaedic surgeon. More recently, the development and use of robotic-assisted technology[74] has made performing UKA technically less demanding and various studies have reported improved radiographic outcomes, more consistent component placement, and fewer outliers[26] (Figure 4).
For example, Bellemans et al. reported implant positioning and alignment to fall within 1° error of neutral alignment for all cases performed with robotic-assistance.[75] Furthermore, Cobb et al. demonstrated that the number of radiographic outliers following UKA decrease significantly with the aid of robotic systems.[26] However, the results of this study should be interpreted with caution, as it remains unclear if these more favorable radiographic outcome measures correlate with greater functional improvement.[26,76 ]
Computer assisted surgery systems, also called passive surgery systems, monitor operative procedures and allow for intraoperative assessment and feedback during adult reconstructive surgery (Figure 5).[67,74,77]
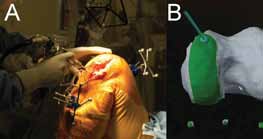
Fig. 5 Example of a system that uses a robotic arm with a high speed burr and gives the surgeon tactile feedback (A) when the planned resection depth is reached (B).74 |
The individual design of passive surgery systems is proprietary, however, these systems track various parameters (i.e. component positioning, bone geometry) during operative procedures.[78] Computer-based intraoperative systems may offer greater accuracy over conventional templating methods, thus, passive systems may be utilized during UKA to more accurately and precisely place components.[66,77,78] Better component placement during UKA has been associated with clinical success.[37,67,77, 78,79] Specifically, Pearle et al. and Cobb et al. found that intraoperative computerguidance enabled component positioning to within 2° of the preoperative plan in all cases.[26,37] In these studies, up to 60% of UKA components were determined to be improperly positioned when computer navigation was not used.[26,37] Other studies have reported that femoral and tibial component alignment, tibial slope, and lower extremity mechanical axis was improved when passive surgery systems were used during UKA.[80-85]
Robotic systems have also been designed to aid surgeons during UKA. Using templates prepared from a computer-tomography scan, the robot provides both tactile and haptic response during the procedure in order to assist the surgeon in matching their preoperative plan. Ligament balancing and range of motion are also obtained intraoperatively with the UKA prosthesis in place. Early reports of radiographic outcomes have been promising when the robotic system has been utilized.[61,86,87] Lonner et al. conducted a radiographic comparison of 31 consecutive patients who underwent roboticassisted UKA to 27 consecutive patients who underwent manual UKA.[86] The authors found that there was almost three times greater variation in tibial component using the standard method, suggesting that robotic-assisted surgery leads to more consistent component placement. However, these findings have not been correlated to clinical outcomes. Additionally, Roche et al. reported on the one-year outcomes of 223 robot-assisted UKAs.[87] At the most recent follow-up, none of the patients required revision surgery and there was a statistically significant improvement in clinical outcome scores. However, until mid-term results are available, many institutions will find it hard to invest in this new technology.
Conclusion
UKA has the potential to become the preferred operation for the treatment of limited degenerative knee disease. With robotic assistance, UKA component placement may become more accurate and precise. Data relating improved short-term radiographic outcomes to enhanced functional outcomes and improved patient satisfaction is limited; thus more studies with longer followup are required before UKA is more widely performed. Favorable outcomes using new robotic technology may encourage orthopaedic surgeons to offer their patients UKA as a treatment option for unicompartmental joint pathology. However, midterm and long-term data are not currently available for robotic-assisted UKA and further investigations are needed.
References
- Kurtz SM. The Origins and Adaptations of UHMWPE for Knee Replacement, Biomaterials Handbook, Chapter 7, Marcel Dekker, Inc. 1995
- Hardinge K. Revision of total knee arthroplasty, Chapter 18.2 in Knee Surgery Current Practice, Martin Duntz Limited 1992
- Minns RJ, Hardinge K. Failure of one design of surface replacement knee arthroplasty due to loosening deformation and wear of the plastic femoral component. Biomaterials, 4(3): 147-52, 1983.
- Bert JM. Unicompartmental knee replacement. Orthop Clin North Am, 36(4): 513-22, 2005.
- Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am, 91 Suppl 1: 63-8, 2009.
- Lonner JH. Patellofemoral Arthroplasty. J Am Acad Orthop Surg, 15(8): 495-506, 2007.
- Product Development, Bechtol; www.jisrf.org.
- Cannon A, Stolley M, Wolf B, Amendola A. Patellofemoral resurfacing arthroplasty: literature review and description of a novel technique. The Iowa orthopaedic journal, 28: 42-8, 2008.
- Laskin RS. Unicompartmental tibiofemoral resurfacing arthroplasty. J Bone Joint Surg Am, 60(2): 182-5, 1978.
- Pearle AD. Kendoff D, Stueber V, Musahl V, Repicci JA. Perioperative management of unicompartmental knee arthroplasty using the MAKO robotic arm system (MAKOplasty). Am J Orthop (Belle Mead NJ), 38(2 Suppl): 16-9, 2009.
- Insall J, Aglietti P. A five to seven-year follow-up of unicondylar arthroplasty. J Bone Joint Surg Am, 62(8): 1329-37, 1980.
- Richards Manufacturing Co., 1975 Corp. Announcement “IT IS WITH A DEEP SENSE OF OBLIGATION TO YOU ...”.
- Vossler v. Richards Manufacturing Co. (1983) 143 Cal.App.3d 952 [192 Cal.Rptr. 219].
- Treace v. Marmor, 644 F.2d 887 (6th Cir. 01/08/1981) HARRY T. TREACE AND RICHARD’S MANUFACTURING COMPANY, INC., PLAINTIFFS-APPELLANTS, v. LEONARD MARMOR, DEFENDANT-APPELLEE.
- Bonutti PM, Dethmers DA. Contemporary unicompartmental knee arthroplasty: fixed vs mobile bearing. J Arthroplasty, 23(7 Suppl): 24-7, 2008.
- Marmor L. The modular knee. Clin Orthop Relat Res, (94): 242-8, 1973.
- Marmor L. Surgical insertion of the modular knee. RN, 36(9): OR1-6, 1973.
- Tanavalee A, Choi YJ, Tria AJ. Unicondylar knee arthroplasty: past and present. Orthopedics, 28(12): 1423-33; quiz 1434-5, 2005.
- Marmor L. The Modular (Marmor) knee: case report with a minimum follow-up of 2 years. Clin Orthop Relat Res, (120): 86-94, 1976.
- Marmor L. Unicompartmental knee arthroplasty. Ten- to 13-year follow-up study. Clin Orthop Relat Res, (226): 14-20, 1988.
- Marmor L. Unicompartmental arthroplasty of the knee with a minimum ten-year follow-up period. Clin Orthop Relat Res, (228): 171-7, 1988.
- Ryd L, Lindstrand A, Stenstrom A, Selvik G. Cold flow reduced by metal backing. An in vivo roentgen stereophotogrammetric analysis of unicompartmental tibial components. Acta orthopaedica Scandinavica, 61(1): 21-5, 1990.
- Broughton NS, Newman JH, Baily RA. Unicompartmental replacement and high tibial osteotomy for osteoarthritis of the knee. A comparative study after 5-10 years’ follow-up. J Bone Joint Surg Br, 68(3): 447-52, 1986.
- Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg Br, 80(6): 983-9, 1998.
- Bert JM. 10-year survivorship of metal-backed, unicompartmental arthroplasty. J Arthroplasty, 13(8): 901-5, 1998.
- Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F, Barrett A, Davies B. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br, 88(2): 188-97, 2006.
- Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg, 16(1): 9-18, 2008.
- Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop (Belle Mead NJ), 38(2 Suppl): 3-6, 2009.
- Grelsamer RP. Unicompartmental osteoarthrosis of the knee. J Bone Joint Surg Am, 77(2): 278-92, 1995.
- Kozinn SC, Marx C, Scott RD. Unicompartmental knee arthroplasty. A 4.5-6-year follow-up study with a metal-backed tibial component. J Arthroplasty, 4 Suppl: S1-10, 1989.
- Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am, 71(1): 145-50, 1989.
- Berend KR, Lombardi AV Jr, Mallory TH, Adams JB, Groseth KL. Early failure of minimally invasive unicompartmental knee arthroplasty is associated with obesity. Clin Orthop Relat Res, 440: 60-6, 2005.
- Heck DA, Marmor L, Gibson A, Rougraff BT. Unicompartmental knee arthroplasty. A multicenter investigation with long-term follow-up evaluation. Clin Orthop Relat Res, (286): 154-9, 1993.
- Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Unicompartmental knee arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am, 85-A(10): 1968-73, 2003.
- Lonner JH. Introduction: robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop (Belle Mead NJ), 38(2 Suppl): 2, 2009.
- Lonner JH. Robotic Arm-Assisted Unicompartmental Arthroplasty. Seminars in Arthroplasty, 20(1): 15-22, 2009.
- Pearle AD, Kendoff D, Musahl V. Perspectives on computer-assisted orthopaedic surgery: movement toward quantitative orthopaedic surgery. J Bone Joint Surg Am, 91 Suppl 1: 7-12, 2009.
- Stern SH, Becker MW, Insall JN. Unicondylar knee arthroplasty. An evaluation of selection criteria. Clin Orthop Relat Res, (286): 143-8, 1993.
- Laskin RS. Unicompartmental knee replacement: some unanswered questions. Clin Orthop Relat Res, (392): 267-71, 2001.
- Beard DJ, Pandit H, Ostlere S, Jenkins C, Dodd CA. and Murray, D. W.: Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg Br, 89(12): 1602-7, 2007.
- Berend KR, Lombardi AV Jr, Morris MJ, Hurst JM, Kavolus JJ. Does preoperative patellofemoral joint state affect medial unicompartmental arthroplasty survival? Orthopedics, 34(9): e494-6, 2011.
- Pandit H, Jenkins C, Gill HS, Smith G, Price AJ, Dodd CA, Murray DW. Unnecessary contraindications for mobile-bearing unicompartmental knee replacement. J Bone Joint Surg Br, 93(5): 622-8, 2011.
- Goodfellow JW, O’Connor J. Clinical results of the Oxford knee. Surface arthroplasty of the tibiofemoral joint with a meniscal bearing prosthesis. Clin Orthop Relat Res, (205): 21-42, 1986.
- Beard DJ, Pandit H, Gill HS, Hollinghurst D, Dodd CA, Murray DW. The influence of the presence and severity of pre-existing patellofemoral degenerative changes on the outcome of the Oxford medial unicompartmental knee replacement. J Bone Joint Surg Br, 89(12): 1597-601, 2007.
- Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty, 25(2): 230-7, 2010.
- Roche M, O’Loughlin PF, Kendoff D, Musahl V, Pearle AD. Robotic arm-assisted unicompartmental knee arthroplasty: preoperative planning and surgical technique. Am J Orthop (Belle Mead NJ), 38(2 Suppl): 10-5, 2009.
- Hoffman M. MAKO Robotics Yearly Numbers for Unicompartmenal Knee Arthroplasty. Edited by Floyd, A. J., Fort Wayne, 2010.
- Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplasty, 23(3): 408-12, 2008.
- The Norwegian Arthroplasty Register. Edited, Helse-Bergen HF, Department of Orthopaedic Surgery, Haukeland University Hospital, 2010.
- Australian Orthopaedic Association National Joint Replacement Registry. In Annual Report. Edited by Association, A. O., Adelaide, 2010.
- Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop (Belle Mead NJ), 38(2 Suppl): 32-6, 2009.
- Jenny JY, Ciobanu E, Boeri C. The rationale for navigated minimally invasive unicompartmental knee replacement. Clin Orthop Relat Res, 463: 58-62, 2007.
- Newman JH, Ackroyd CE, Shah NA. Unicompartmental or total knee replacement? Five-year results of a prospective, randomised trial of 102 osteoarthritic knees with unicompartmental arthritis. J Bone Joint Surg Br, 80(5): 862-5, 1998.
- Bert J. Analysis of unicompartmental arthroplasty in a community based registry. In American Academy of Orthopedic Surgeons. Edited, Dallas, TX, 2002.
- Kalairajah Y, Simpson D, Cossey AJ, Verrall GM, Spriggins AJ. Blood loss after total knee replacement: effects of computer-assisted surgery. J Bone Joint Surg Br, 87(11): 1480-2, 2005.
- Hinarejos P, Corrales M, Matamalas A, Bisbe E, Caceres E. Computer-assisted surgery can reduce blood loss after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc, 17(4): 356-60, 2009.
- Laurencin CT, Zelicof SB, Scott RD, Ewald FC. Unicompartmental versus total knee arthroplasty in the same patient. A comparative study. Clin Orthop Relat Res, (273): 151-6, 1991.
- Dalury DF, Fisher DA, Adams MJ, Gonzales RA. Unicompartmental knee arthroplasty compares favorably to total knee arthroplasty in the same patient. Orthopedics, 32(4), 2009.
- Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am, 87(2): 332-8, 2005.
- Hopper GP, Leach WJ. Participation in sporting activities following knee replacement: total versus unicompartmental. Knee Surg Sports Traumatol Arthrosc, 16(10): 973-9, 2008.
- Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop (Belle Mead NJ), 38(2 Suppl): 20-2, 2009.
- Banks SA, Harman MK, Hodge WA. Mechanism of anterior impingement damage in total knee arthroplasty. J Bone Joint Surg Am, 84-A Suppl 2: 37-42, 2002.
- Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res, (423): 161-5, 2004.
- Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am, 86-A(3): 506-11, 2004.
- Li G, Papannagari R, Most E, Park SE, Johnson T, Tanamal L, Rubash HE. Anterior tibial post impingement in a posterior stabilized total knee arthroplasty. J Orthop Res, 23(3): 536-41, 2005.
- Mariani EM, Bourne MH, Jackson, RT, Jackson ST, Jones P. Early failure of unicompartmental knee arthroplasty. J Arthroplasty, 22(6 Suppl 2): 81-4, 2007.
- Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty, 22(7): 1054-9, 2007.
- Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty, 20(4 Suppl 2): 2-3, 2005.
- Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am, 69(9): 1328-35, 1987.
- Kasodekar VB, Yeo SJ, Othman S. Clinical outcome of unicompartmental knee arthroplasty and influence of alignment on prosthesis survival rate. Singapore Med J, 47(9): 796-802, 2006.
- Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty, 21(6 Suppl 2): 108-15, 2006.
- Jeer PJ, Keene GC, Gill P. Unicompartmental knee arthroplasty: an intermediate report of survivorship after the introduction of a new system with analysis of failures. Knee, 11(5): 369-74, 2004.
- Ridgeway SR, McAuley JP, Ammeen DJ, Engh GA. The effect of alignment of the knee on the outcome of unicompartmental knee replacement. J Bone Joint Surg Br, 84(3): 351-5, 2002.
- Lang JE, Mannava S, Floyd AJ, Goddard MS, Smith BP, Mofidi A, Seyler TM, Jinnah RH. Robotic systems in orthopaedic surgery. J Bone Joint Surg Br, 93(10): 1296-9, 2011.
- Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res, 464: 111-6, 2007.
- Jenny JY, Boeri C. Computer-assisted implantation of total knee prostheses: a case-control comparative study with classical instrumentation. Comput Aided Surg, 6(4): 217-20, 2001.
- Jenny JY, Boeri C. Unicompartmental knee prosthesis implantation with a non-image-based navigation system: rationale, technique, case-control comparative study with a conventional instrumented implantation. Knee Surg Sports Traumatol Arthrosc, 11(1): 40-5, 2003.
- Davies B, Rodriguez y Baena F, Barrett A, Gomes M, Harris S, Jakopec M, Cobb, J. Robotic control in knee joint replacement surgery. Proc Inst Mech Eng H, 221(1): 71-80, 2007.
- Keene G, Simpson D, Kalairajah Y. Limb alignment in computer-assisted minimally-invasive unicompartmental knee replacement. J Bone Joint Surg Br, 88(1): 44-8, 2006.
- Bathis H, Perlick L, Luring C, Kalteis T, Grifka J. [CT-based and CT-free navigation in knee prosthesis implantation. Results of a prospective study]. Der Unfallchirurg, 106(11): 935-40, 2003.
- Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop, 2010.
- Decking R, Markmann Y, Fuchs J, Puhl W, Scharf HP. Leg axis after computer-navigated total knee arthroplasty: a prospective randomized trial comparing computer-navigated and manual implantation. J Arthroplasty, 20(3): 282-8, 2005.
- Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer-assisted total knee arthroplasty using patient-specific templating. Clin Orthop Relat Res, 444: 184-92, 2006.
- Jenny JY, Clemens U, Kohler S, Kiefer H, Konermann W, Miehlke RK. Consistency of implantation of a total knee arthroplasty with a non-image-based navigation system: a case-control study of 235 cases compared with 235 conventionally implanted prostheses. J Arthroplasty, 20(7): 832-9, 2005.
- Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty, 22(8): 1097-106, 2007.
- Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res, 468(1): 141-6, 2010.
- Roche MW, Augustin D, Conditt MA. One Year Outcomes of Robotically Guided UKA. J Bone Joint Surg Br, 92-B(SUPP_I): 156-b-157, 2010.
|

